Ulbrich Expands Braid Wire Accelerator to Fast Track Medical Device Development

Westminster, SC – When sourcing materials for the manufacturing of critical medical devices, speed is everything. The faster you can procure desired materials, the sooner you can start testing engineered applications. And the quicker you begin iterating, the greater the chance your solution beats your competitors to market.
Freudenberg Medical Receives Edwards Lifesciences Supplier Award

Freudenberg Medical Asia is proud to have received a Silver Recognition Supplier Award from our long-standing customer, Edwards Lifesciences.
Wearable and implantable medical devices tap humans as a power source

Wearable medical devices and implants are becoming more prominent in a wide range of applications such as health monitoring devices and biomedical implants, which provide continuous measurements of biomarkers and medical diagnostics.
First IVDR for Class D Medical Device
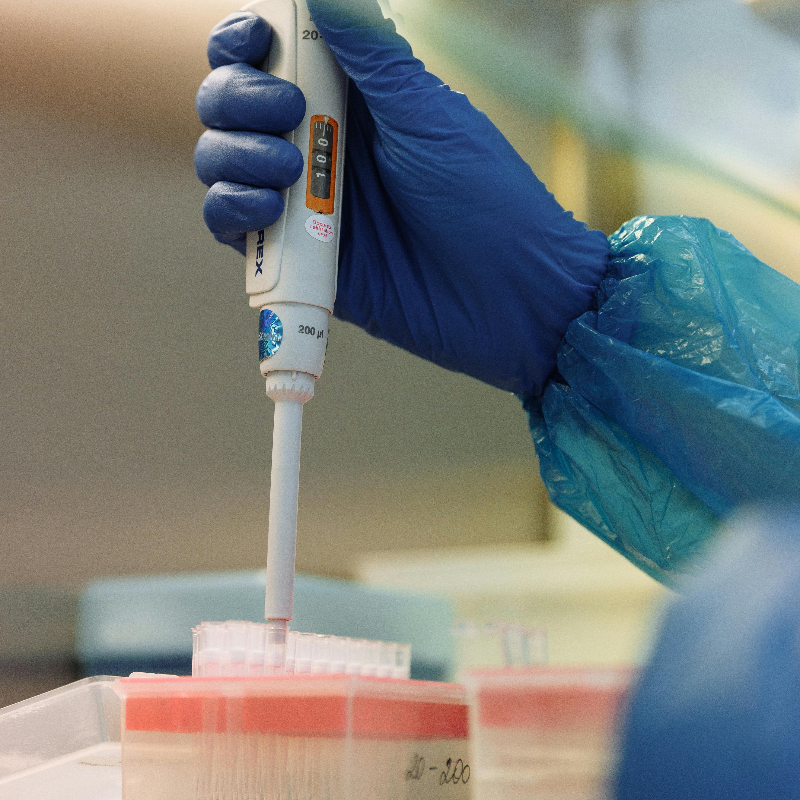
Roche Diagnostics is the first manufacturer to score certification for a class D product under the new in vitro diagnostic regulation (IVDR).
Surgical Navigation: Breakthrough Innovations in 6DOF Expand Potential for Minimally Invasive Diagnostic and Therapeutic Devices

Whether it’s robotic navigated surgery or interventional imaging and tracking, less invasive procedures both diagnostic and therapeutic in nature could benefit cardiology and electrophysiology, endoscopy and more.
Could New Imaging System Reduce the Need for Some Breast Biopsies
Each year, 30 million women in the United States undergo screening mammograms, leading to six and a half million of those women requiring more than 17 million additional diagnostic procedures.


